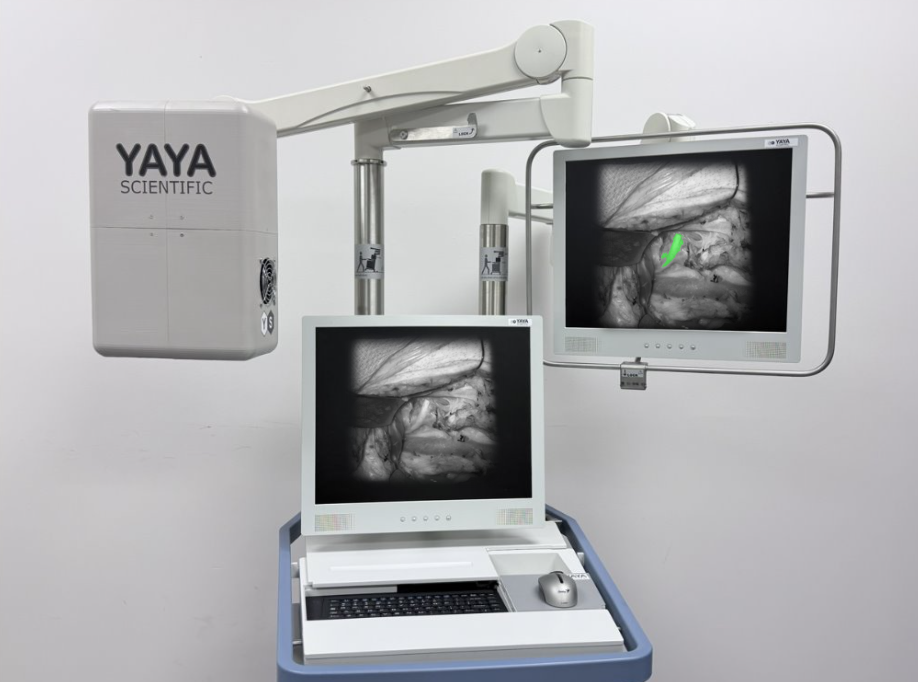
The Journey Behind Alina™
As we look back on the path that brought us to 2025, it’s remarkable to see how far YaYa Scientific has come. What began as a clinical question from surgeons at Vanderbilt University Medical Center has now grown into Alina™, our real-time, label-free, non-contact nerve-identification system — and this year, for the first time, Alina™ stepped into the operating room.
This milestone has been years in the making. And in many ways, we are still at the beginning of what this technology can become.
Where It All Started (2017–2018)
Our journey began in 2017 and 2018, when surgeons from three different departments at Vanderbilt — Neuro-Oncology, Surgical Oncology & Endocrine Surgery, and General Surgery — independently approached us with the same challenge:
They needed a better way to identify nerves during surgery.
Despite advances in imaging and surgical tools, there was still no reliable, real-time, dye-free solution available to help surgeons distinguish nerves from surrounding tissue. After speaking with teams across specialties, it became clear that this was not a niche request — it was a meaningful unmet need.
We began exploring whether combining polarization imaging with diffuse reflectance spectroscopy could provide surgeons with actionable information during procedures. Using an early setup, we collected preliminary lab data and saw approximately 85% accuracy in nerve identification through post-processing. Even at this early stage, we knew the concept had real potential.
Becoming YaYa Scientific (2019)
Encouraged by early findings and supported by strong clinical interest, we founded YaYa Scientific in March 2019. The mission was ambitious but clear:
Develop a real-time, label-free intraoperative imaging system that helps surgeons protect nerves and improve patient outcomes.
This idea would ultimately become Alina™.
Turning a Concept Into a Device (2021–2024)
A major turning point came in 2021 when we were awarded a Phase I STTR grant from NIH–NIBIB. This support enabled us to conduct preclinical studies, including an IACUC-approved rat sciatic nerve model where the underlying technology performed with greater than 95% nerve-identification accuracy in real time.
These results led to a Phase II STTR award and allowed us to begin transforming our benchtop research system into an alpha clinical prototype of Alina™ suitable for use in humans.
Throughout 2024 and into 2025, our team worked closely with surgeons and engineers to refine the system.
We focused on:
- Enhancing optical performance
- Strengthening computational accuracy
- Improving usability and ergonomics
- Ensuring compatibility with surgical workflow
During this period, Alina™ matured from an early experimental setup into a thoughtfully engineered clinical device.
Entering the Operating Room (2025)
In September 2025, Alina™ entered the operating room for the first time as part of our initial human clinical pilot study at the John Hopkins University School of Medicine and the Vanderbilt University Medical Center.
This milestone marked the culmination of our Phase II development efforts. The pilot study will help us:
- Establish baseline nerve-identification performance in human subjects
- Understand system behavior in real surgical environments
- Gather data needed to design our upcoming clinical trial
- Validate the clinical utility of our imaging approach
Seeing Alina™ in use during live procedures was an important moment for our entire team — a reminder of why we began this work in the first place.
What Comes Next: Beta Development (2026)
As we look ahead, early 2026 marks the start of beta prototype development for Alina™. This next phase will build on everything we’ve learned over the past several years and focus on creating a device ready for broader clinical evaluation.
Our beta development priorities include:
- Integrating insights from the 2025 pilot study
- Refining hardware and software for greater robustness
- Enhancing workflow efficiency and user experience
- Advancing industrial design and overall system architecture
- Preparing for multi-center clinical trials
- Establishing the foundation for future regulatory pathways
The beta version of Alina™ will represent the most advanced form of our technology to date — designed for reliability, usability, and clinical scalability.
Looking Forward With Gratitude
Reflecting on our journey from 2017 to 2025, one thing stands out: this progress was only possible because of the collaboration and support of many people. Surgeons, engineers, scientists, advisors, and investors have all played critical roles in shaping Alina™.
As we enter 2026, our mission remains steady:
Provide surgeons with a real-time, label-free nerve-identification system that enhances surgical precision and protects patient quality of life.
Reaching the operating room was a major step. Building the beta prototype and preparing for multi-center trials will be the next. The path forward is exciting, and we look forward to sharing each step as Alina™ continues to evolve.
